Supramolecular chemistry involves the organization of molecules into larger, higher-order structures. When suitable building blocks are chosen, these systems ‘self-assemble’ from their individual components.
Certain supramolecular compounds are well suited for ‘host-guest chemistry’. In such cases, a host structure encloses a guest molecule and can shield, protect and transport it away from its environment. This is a specialist field of Dr. Bernd M. Schmidt and his research group at the Institute of Organic and Macromolecular Chemistry at HHU.
The chemists in Düsseldorf collaborated with colleagues from the DWI Leibniz Institute for Interactive Materials to find a system that may one day even be able to transport cargo molecules through the human body and release the drug at the desired location.
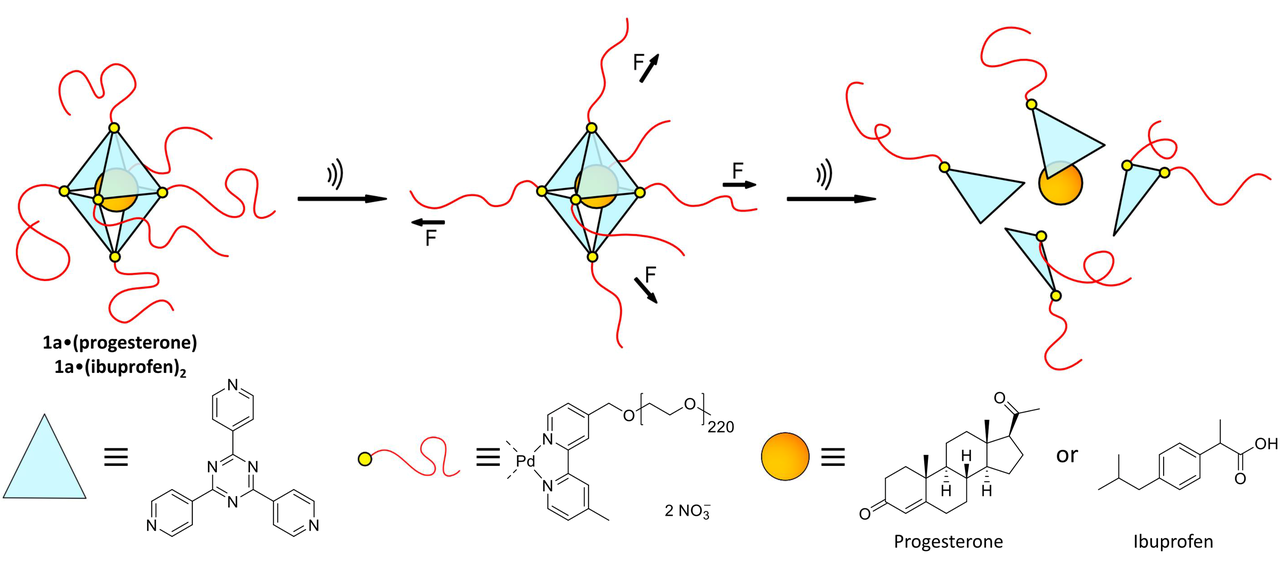
The solution may be to use discrete ‘Pd6(TPT)4 cages’. These are octahedral cage-like assemblies, bearing polymer chains on each vertex. They are comprised of four triangular panels, palladium atoms and connecting units.
When the individual components are added to an aqueous solution in the correct ratio, the cages self-assemble. If smaller, hydrophobic molecules are added to the cages, they enter the cavities. The researchers demonstrated this effect using pharmaceutically active molecules, like ibuprofen and progesterone.
“The special trick with our system involves the pre-determined rupture points”, explains Dr. Schmidt, last author of the study. “The palladium atoms hold all compounds with a comparatively weak bond. Once you succeed in breaking the atoms out of the compound, the entire octohedral structure breaks apart.”
To break the bonds, the researchers in Aachen use powerful ultrasonification similar to that used medically to break down kidney stones, for example. In water, the ultrasound creates cavitation bubbles that burst and exert huge mechanical shear force on the long polymer chains. The forces are so powerful that the palladium atoms are actually torn from the vertices and thus rupture the octahedral cage. The small drug molecules are agitated in the process but are not damaged.
Dr. Robert Göstl (DWI) says: “Localised ultrasound radiation of the tissue to be treated could mean that the drug transported in the cage is later released at the exact location where the therapy is needed.” The drug molecules used in the study serve merely as examples. In principle, a large number of different hydrophobic molecules can be packed in the cage. Unlike other host-guest systems described, it is not necessary to alter the drug molecules chemically in order to get them in the cage. “To treat tumours, it would be feasible to use cytostatic drugs as the cargo, for example. By releasing them directly at the site of a solid tumour, it may be possible to have chemotherapy that uses much less of the drug and thus has lesser side effects”, explains Schmidt.
This is helped by the fact that the defined cargo volume makes it possible to measure precisely how much of the drug is released at the target site. “The dose administered could even be calculated precisely.”
The study is a Proof of Concept that demonstrated the feasibility of the approach. It also convinced the reviewers and publishers of the journal “Angewandte Chemie”, who rated the publication as very important. The work, which is classified as “Hot Paper”, will also be featured on the cover of the upcoming issue.
“The next steps involve determining how real cells respond to our cages. Before any medical use, we need to ensure that they are not toxic.”
Original publication
Robin Küng, Tobias Pausch, Dustin Rasch, Robert Göstl und Bernd M. Schmidt, Mechanochemical Release of Non-Covalently Bound Guests from a Polymer-Decorated Supramolecular Cage, Angew. Chem. Int. Ed. (2021)